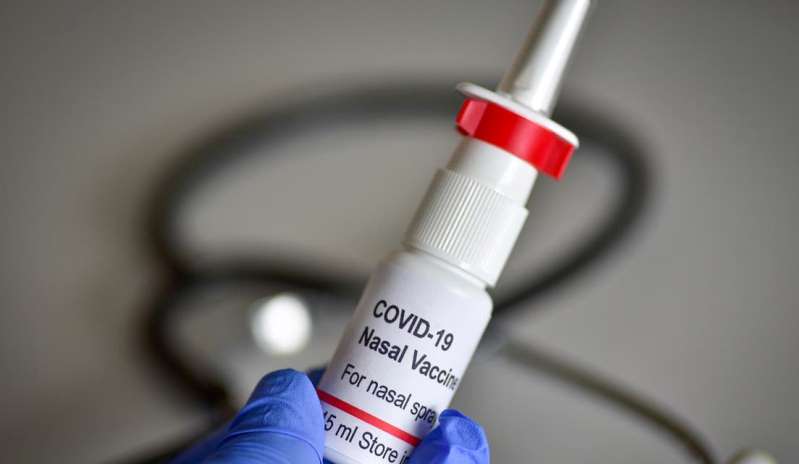
The Drugs Controller General of India (DCGI) granted permission to Bharat Biotech to start conducting booster dose trials for its intranasal coronavirus vaccine. The approval has been granted to conduct phase-3 trials. The trials will assess the nasal vaccine for both the dose and use it as a booster dose schedule, the company said.
The trials will be conducted at nine different sites by Bharat Biotech. The company had received regulatory approval for mid-to-late-stage trials for its intranasal vaccine BBV154 in August last year. The vaccine maker submitted the late-stage trial application to the DGCI in December 2021, saying that an intranasal vaccine as a booster dose will be easier to give in mass vaccination campaigns.
 Live
Live