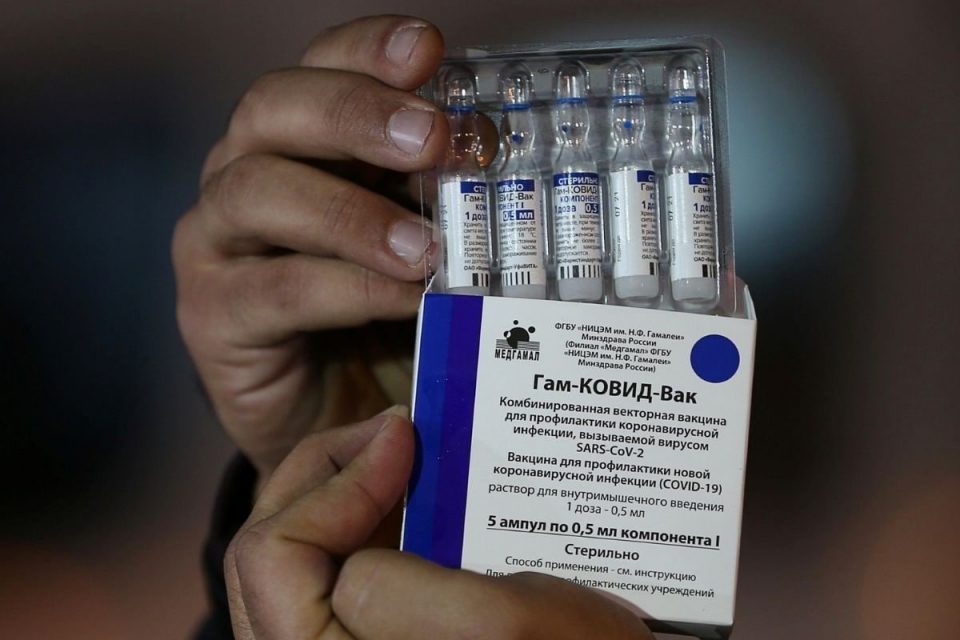
The Drug Controller General of India (DCGI) has approved the restricted emergency use of the Sputnik V vaccine late Monday night. With this, India now has three vaccines against COVID-19, including Covishield the Oxford-AstraZeneca vaccine manufactured by the Serum Institute of India, and Bharat Biotech’s Covaxin According to a release issued by the Russian Direct Investment Fund (RDIF), India is the most populated country to register the Russian vaccine. “Total population of 60 countries where Sputnik-V is approved for use is 3 billion people or about 40% of the global population,” it added.
- NASA Reports Fuel Leak in Moon Rocket Test Countdown
- SC Slams WhatsApp Over Data Sharing for Ads
- India Unlikely to Halt Russian Oil Imports: Ex-Foreign Secretary
- Godrej Properties Rallies 9.75% on Rs 1,000 Cr Sales
- KEC International Bags Rs 1,020 Crore Orders; Shares Rally 5%
The vaccine has been registered in India under the emergency use authorization procedure based on results of clinical trials in Russia as well as positive data of additional Phase III local clinical trials in India conducted in partnership with Dr. Reddy’s Laboratories, it added. “India is the leading production hub for Sputnik-V. RDIF has reached agreements with the leading pharmaceutical companies in the country (Gland Pharma, HeteroBiopharma, Panacea Biotec, Stelis Biopharma, Virchow Biotech) aimed at the production of more than 850 million doses per year,” noted the release.
 Live
Live