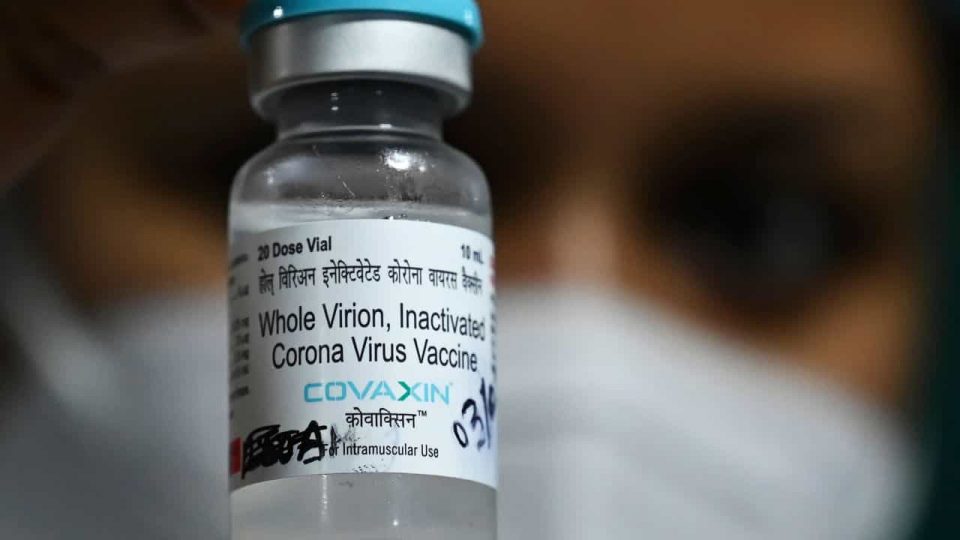
As the threat of the third wave of Covid-19 appears large over India, the AIIMS Chief expects approval of the Covaxin coronavirus vaccine for children by October. AIIMS Director Dr Randeep Guleria said that Bharat Biotech is expected to submit clinical trials on children by September-October. The pandemic can be controlled only if everyone is vaccinated, he said.
“Bharat Biotech and other companies are doing trials at a breakneck speed as parents have come forward with their children for the trials,” he said. “One is hopeful that the trial will be completed early and possibly with follow up of about two-three months, we shall have data by September.
- ChatGPT Mobile App Introduces Video and Screensharing Features
- India’s Forex Reserves Drop by $3.23 Billion to $654.86 Billion on 6th Dec
- Paraguayan President Santiago Pena Opens Jerusalem Embassy
- Premier Energies Planning to Establish 1 GW Manufacturing Plant in Telangana
- International Gemmological Institute (India) IPO GMP, Lot Size & Key Dates
Hopefully, by that time, vaccines will be approved so that by September-October, we can give to children,” he added.
He further said that the Covaxin “covers a wide spectrum” as Bharat Biotech conducts trials between the age group of 2 to 18 years. Covaxin, developed by Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR), is being used on adults in India’s current vaccination drive.