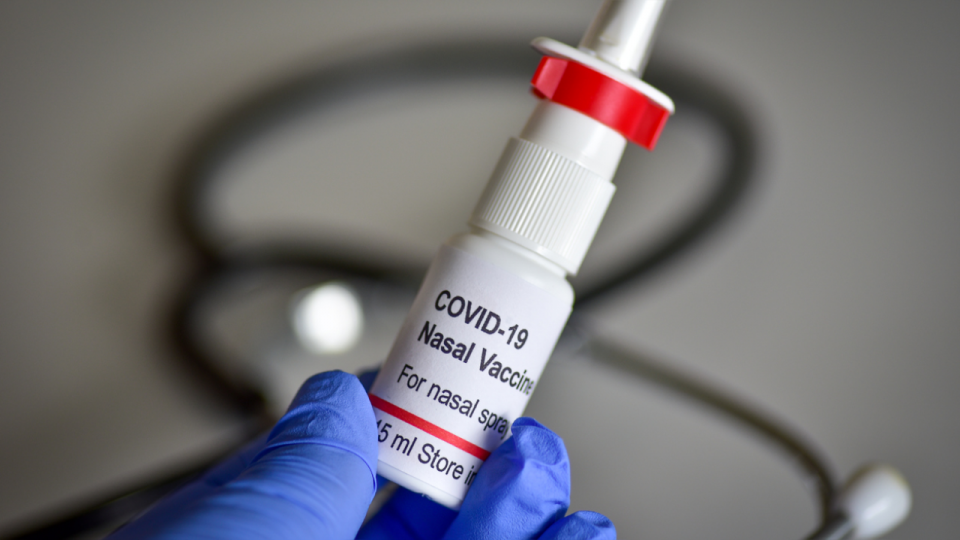
Bharat Biotech has got a nod from the Central Drugs Standard Control Organisation(CDSCO) to conduct Phase 2/3 trials of its nasal Covid-19 vaccine. Phase 1 trial of the intranasal vaccine in volunteers aged 18 to 60 years was well tolerated, the science and technology ministry said in a release.
BBV154 is the first intranasal Covid-19 vaccine to go through human trials in India. It has got permission to conduct a Phase 2 clinical trial of “heterologous prime-boost combination of Sars-CoV-2 vaccines to decide the immunogenicity and safety of Covaxin with BBV154 in volunteers”, the ministry said.
Bharat Biotech developed this vaccine with the Department of Biotechnology (DBT) and Biotechnology Industry Research Assistance Council (BIRAC). They supported fast track research for vaccine development, diagnostics, drug repurposing, therapeutics and testing.
“The department is committed to the development of safe and effective Covid-19 vaccines,” said Renu Swarup, secretary, DBT. She said BBV154 is the first intranasal Covid-19 vaccine made in India, which is entering into last-stage clinical trials. Bharat Biotech’s Covaxin had 77.8 per cent efficiency against Covid-19 and 93.4 per cent against severe illness during the Phase 3 clinical trials.


 Signals, Powered By EquityPandit
Signals, Powered By EquityPandit