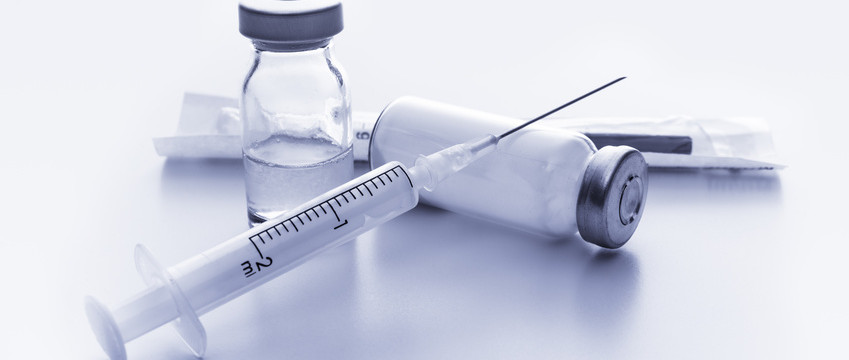
The approval has been received from US Food & Drug Administration (USFDA) for Aurobindo Pharma to manufacture Medroxyprogesterone Acetate Injectable Suspension USP with 150 mg/mL, 1 mL single dose Vial to be bioequivalent and therapeutically equivalent to the reference listed drug (RLD), Depo-Provera Injectable Suspension, of Pfizer Inc.
- Ashoka Buildcon Shares Slump 3% Despite Securing Rs 410 Crore Project
- Zydus Lifesciences Gets USFDA Nod; Stock Trades Flat
- Religare Enterprises Shares Slumped 6% on Demerger Plan
- Natco Pharma Gets CDSCO Nod; Shares Rally 12.5%
- Lodha Developers Buys Solidrise for Rs 300 Cr; Shares Jump 1.5%
In the third quarter of next year, this product will be launched with an estimated market size of around US$ 62 million for the twelve months ending June 2022, according to IQVIA.
This is the 147th ANDA (including ten tentative approvals received) out of Eugia Pharma Speciality Group (EPSG) facilities, manufacturing both oral and sterile speciality products. Medroxyprogesterone Acetate Injection is used to prevent pregnancy for a female’s reproductive potential.
On the other hand, Aurobindo Pharma Limited (APL) ‘s shares were traded at Rs 559.60 close yesterday, which is compared to the previous close of Rs 551.80. The total number of share days was 51246 in over 2236 trades. The company’s stock hits a high of Rs 560.90 and a low of Rs 546.40 on an intraday basis. During the day, the net turnover was Rs 28488804.
 Live
Live