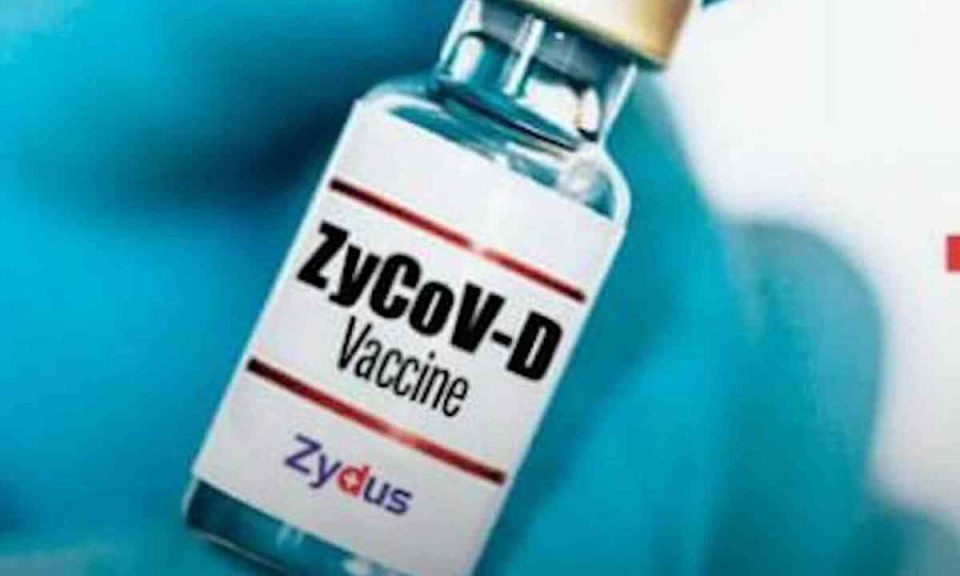
Zydus Cadila‘s Covid-19 vaccine ZyCoV-D is getting a nod for emergency use in India by this week. Union Health Minister Mansukh Mandaviya in the Rajya Sabha had said, “The government assumes that by October-November, four more Indian pharma companies will start production of vaccines which will help to reach the demand of vaccines, Biological E and Novartis vaccines will also be available in the coming months, and Zydus Cadila will soon get an emergency use permission from the Expert Committee.”
- Kotak Mahindra Bank Shares Crash 13% Post RBI Action
- Nestle India Joins Hands With Dr Reddy for Nutritional Health Solutions
- Inox Wind Shares Traded 9% Higher On Board Proposal For Bonus Shares Issuance
- Nestle India Stocks Gained 3% on Q4FY24 Business and Financial Updates
- Rail Vikas Nigam Shares Gain 3% on Securing Project Worth Rs 239 Crore
Zydus Cadila had said before that it has appealed for emergency use authorisation (EUA) with the Drug Controller General of India (DCGI) for its vaccine ZyCoV-D. The pharma company has conducted the most extensive clinical trial in India, with over 50 centres. Zydus Cadila’s vaccine will be the sixth coronavirus vaccine approved in India by the drug regulator after Covishield, Covaxin, Sputnik V, Moderna and Johnson & Johnson (J&J).


 Signals, Powered By EquityPandit
Signals, Powered By EquityPandit